Expo
view channel
view channel
view channel
view channel
view channel
Medical Imaging
AICritical CareSurgical TechniquesPatient CareHealth ITPoint of CareBusiness
Events

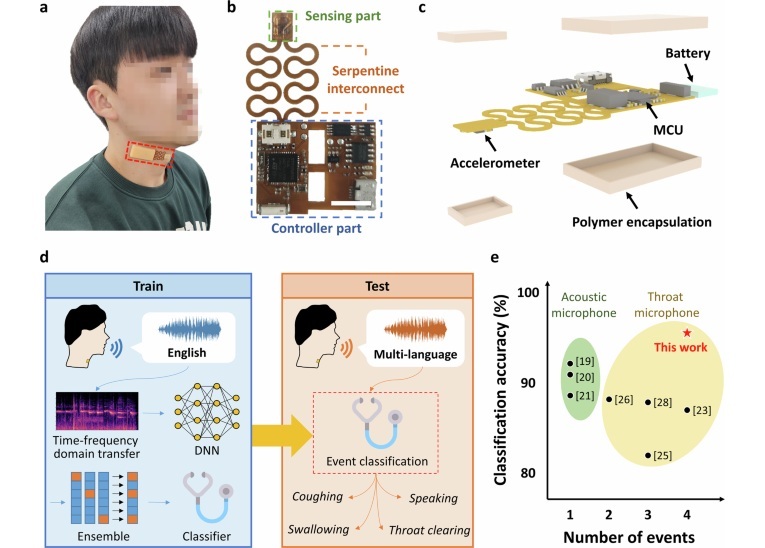
- Battery-Powered Wearable Device Monitors Joint Pain
- Wireless Pacifier Monitors Vitals of NICU Babies Without Need for Painful Blood Draws
- Breakthrough Sensor Technology Tracks Stroke After Effects
- New Study Demonstrates AI-Assisted Detection of Reduced Ejection Fraction
- Novel 3D Adipose Tissue Bioprinting Method to Find Applications in Regenerative Medicine
- Groundbreaking Leadless Pacemaker to Prevent Invasive Surgeries for Children
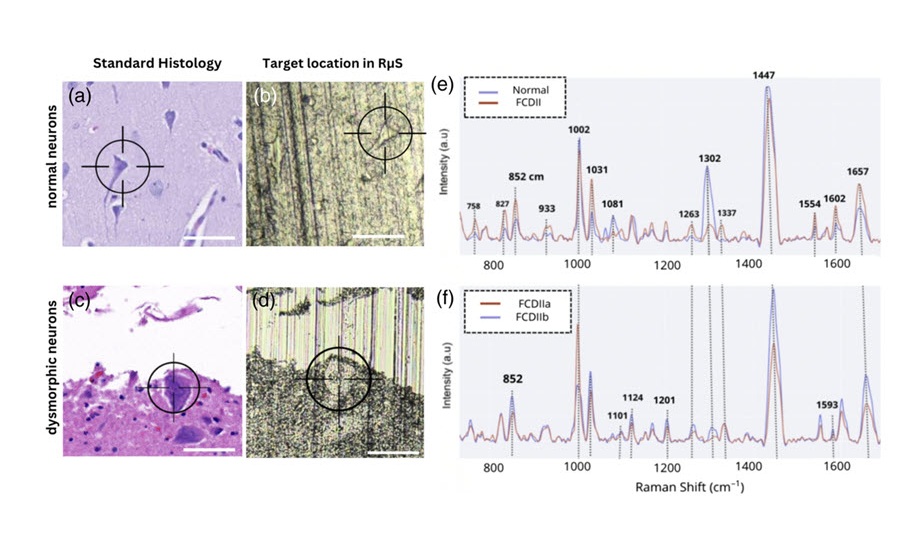
- Spectroscopy Technique Improves Surgery for Pediatric Epilepsy Patients
- Online Tool Guides Surgical Decisions for Gallbladder Cancer
- Bioengineered Arteries Show Promise for Cardiovascular Surgery
- Innovative Technology Enables Rapid Life-Saving Surgical Leak Detection
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Next Gen ICU Bed to Help Address Complex Critical Care Needs
- Groundbreaking AI-Powered UV-C Disinfection Technology Redefines Infection Control Landscape
- Boston Scientific Acquires Medical Device Company SoniVie
- 2026 World Hospital Congress to be Held in Seoul
- Teleflex to Acquire BIOTRONIK’s Vascular Intervention Business
- Philips and Mass General Brigham Collaborate on Improving Patient Care with Live AI-Powered Insights
- Arab Health 2025 Celebrates Landmark 50th Edition
- Smartwatches Could Detect Congestive Heart Failure
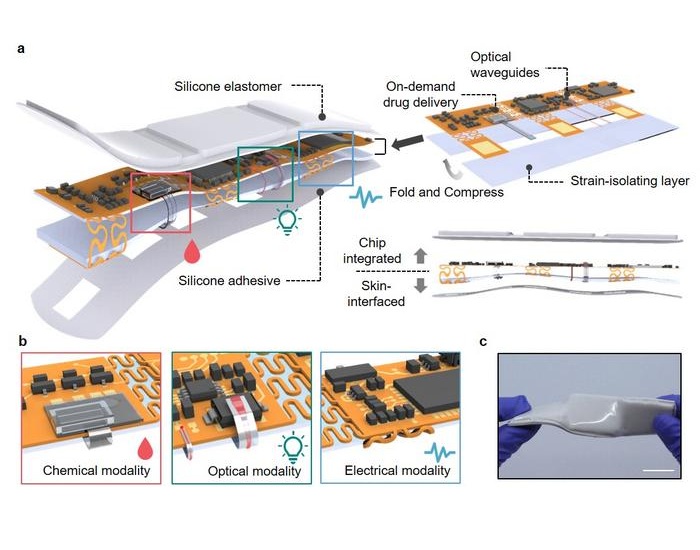
- Versatile Smart Patch Combines Health Monitoring and Drug Delivery
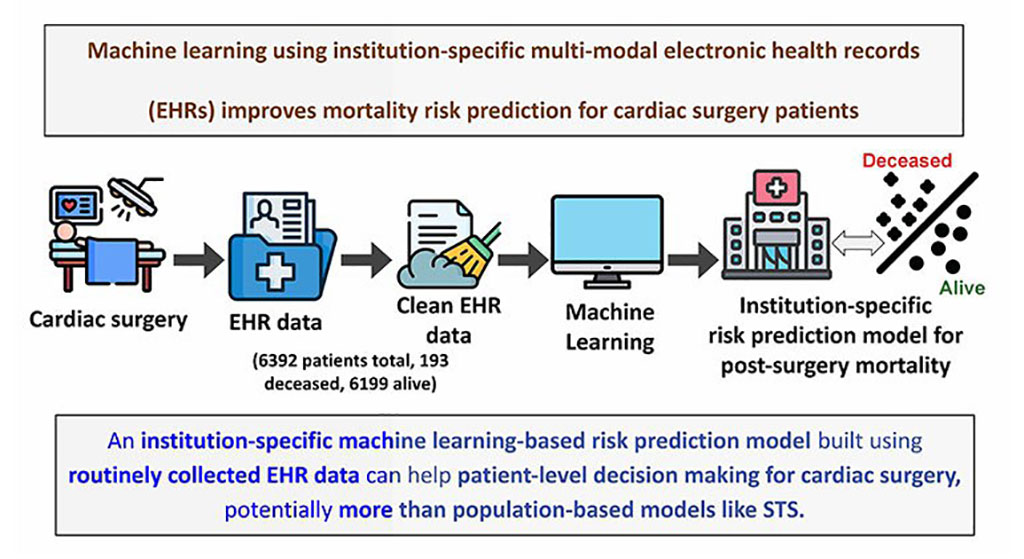
- Machine Learning Model Improves Mortality Risk Prediction for Cardiac Surgery Patients
- Strategic Collaboration to Develop and Integrate Generative AI into Healthcare
- AI-Enabled Operating Rooms Solution Helps Hospitals Maximize Utilization and Unlock Capacity

Expo
 view channel
view channel
view channel
view channel
view channel
Medical Imaging
AICritical CareSurgical TechniquesPatient CareHealth ITPoint of CareBusiness
Events
Advertise with Us
view channel
view channel
view channel
view channel
view channel
Medical Imaging
AICritical CareSurgical TechniquesPatient CareHealth ITPoint of CareBusiness
Events
Advertise with Us


- Battery-Powered Wearable Device Monitors Joint Pain
- Wireless Pacifier Monitors Vitals of NICU Babies Without Need for Painful Blood Draws
- Breakthrough Sensor Technology Tracks Stroke After Effects
- New Study Demonstrates AI-Assisted Detection of Reduced Ejection Fraction
- Novel 3D Adipose Tissue Bioprinting Method to Find Applications in Regenerative Medicine
- Groundbreaking Leadless Pacemaker to Prevent Invasive Surgeries for Children
- Spectroscopy Technique Improves Surgery for Pediatric Epilepsy Patients
- Online Tool Guides Surgical Decisions for Gallbladder Cancer
- Bioengineered Arteries Show Promise for Cardiovascular Surgery
- Innovative Technology Enables Rapid Life-Saving Surgical Leak Detection
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Next Gen ICU Bed to Help Address Complex Critical Care Needs
- Groundbreaking AI-Powered UV-C Disinfection Technology Redefines Infection Control Landscape
- Boston Scientific Acquires Medical Device Company SoniVie
- 2026 World Hospital Congress to be Held in Seoul
- Teleflex to Acquire BIOTRONIK’s Vascular Intervention Business
- Philips and Mass General Brigham Collaborate on Improving Patient Care with Live AI-Powered Insights
- Arab Health 2025 Celebrates Landmark 50th Edition
- Smartwatches Could Detect Congestive Heart Failure
- Versatile Smart Patch Combines Health Monitoring and Drug Delivery
- Machine Learning Model Improves Mortality Risk Prediction for Cardiac Surgery Patients
- Strategic Collaboration to Develop and Integrate Generative AI into Healthcare
- AI-Enabled Operating Rooms Solution Helps Hospitals Maximize Utilization and Unlock Capacity