Expo
view channel
view channel
view channel
view channel
view channel
Medical Imaging
AICritical Care
Patient CareHealth ITPoint of CareBusiness
Events

- Novel ‘Living’ Biomaterial to Advance Regenerative Medicine
- New Cardiovascular Risk Score Uses Stress Test to Predict Heart Disease More Accurately
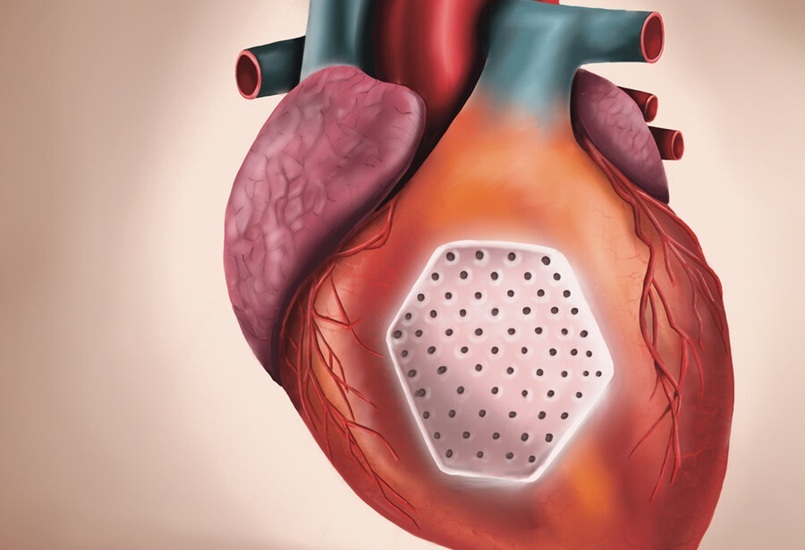
- Heart Patch Offers Innovative Treatment Option for Advanced Heart Failure Patients
- Basal Cell Carcinoma Treatment Reduces Tumor Size, Improves Surgical Removal
- Light-Activated Ink Repairs Heart by Non-Invasively Manipulating Cardiac Tissue Activity
- Innovative Apatite Nanoparticles Improve Biocompatibility of Medical Implants
- Nanotechnology-Based Drug Delivery System Could Help Dialysis and Heart Patients Avoid Repeat Surgeries
- Groundbreaking Tool Accurately Predicts Stroke Outcome for Better Carotid Surgery Decisions
- Pioneering Technology Enables Real-Time Detection of Residual Breast Cancer During Lumpectomy
- Advanced Augmented Reality System to Transform Spine Surgery
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Next Gen ICU Bed to Help Address Complex Critical Care Needs
- Groundbreaking AI-Powered UV-C Disinfection Technology Redefines Infection Control Landscape
- Boston Scientific Acquires Medical Device Company Intera Oncology
- MEDICA 2024 to Highlight Hot Topics of MedTech Industry
- Start-Ups To Once Again Play Starring Role at MEDICA 2024
- Boston Scientific to Acquire AFib Ablation Company Cortex
- Hologic Acquires Gynesonics to Strengthen Existing Gynecological Surgical Business
- Smartwatches Could Detect Congestive Heart Failure
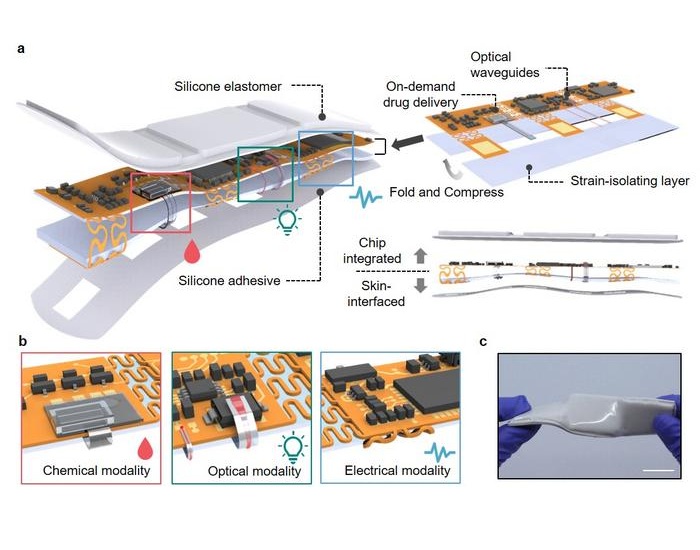
- Versatile Smart Patch Combines Health Monitoring and Drug Delivery
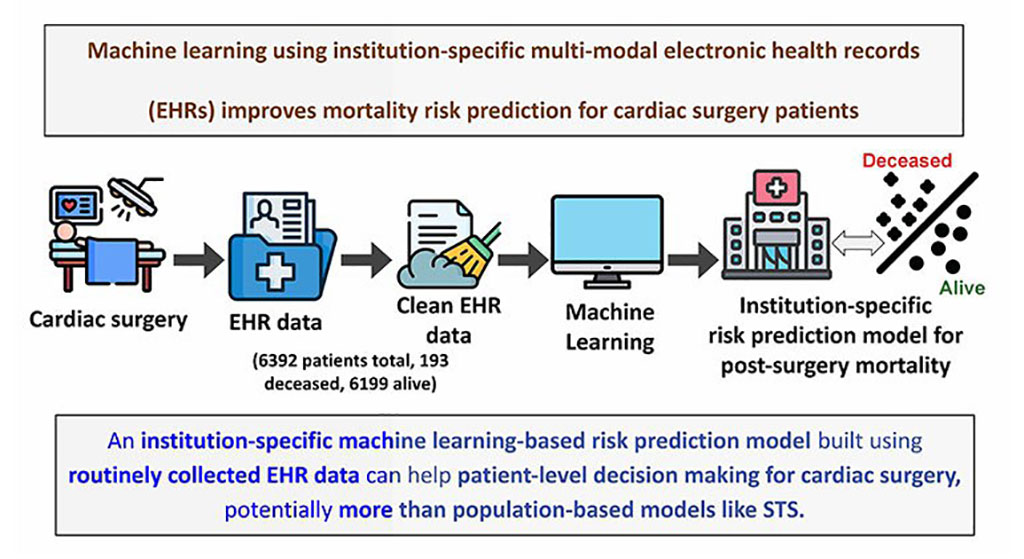
- Machine Learning Model Improves Mortality Risk Prediction for Cardiac Surgery Patients
- Strategic Collaboration to Develop and Integrate Generative AI into Healthcare
- AI-Enabled Operating Rooms Solution Helps Hospitals Maximize Utilization and Unlock Capacity

Expo
 view channel
view channel
view channel
view channel
view channel
Medical Imaging
AICritical Care
Patient CareHealth ITPoint of CareBusiness
Events
Advertise with Us
view channel
view channel
view channel
view channel
view channel
Medical Imaging
AICritical Care
Patient CareHealth ITPoint of CareBusiness
Events
Advertise with Us


- Novel ‘Living’ Biomaterial to Advance Regenerative Medicine
- New Cardiovascular Risk Score Uses Stress Test to Predict Heart Disease More Accurately
- Heart Patch Offers Innovative Treatment Option for Advanced Heart Failure Patients
- Basal Cell Carcinoma Treatment Reduces Tumor Size, Improves Surgical Removal
- Light-Activated Ink Repairs Heart by Non-Invasively Manipulating Cardiac Tissue Activity
- Innovative Apatite Nanoparticles Improve Biocompatibility of Medical Implants
- Nanotechnology-Based Drug Delivery System Could Help Dialysis and Heart Patients Avoid Repeat Surgeries
- Groundbreaking Tool Accurately Predicts Stroke Outcome for Better Carotid Surgery Decisions
- Pioneering Technology Enables Real-Time Detection of Residual Breast Cancer During Lumpectomy
- Advanced Augmented Reality System to Transform Spine Surgery
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Next Gen ICU Bed to Help Address Complex Critical Care Needs
- Groundbreaking AI-Powered UV-C Disinfection Technology Redefines Infection Control Landscape
- Boston Scientific Acquires Medical Device Company Intera Oncology
- MEDICA 2024 to Highlight Hot Topics of MedTech Industry
- Start-Ups To Once Again Play Starring Role at MEDICA 2024
- Boston Scientific to Acquire AFib Ablation Company Cortex
- Hologic Acquires Gynesonics to Strengthen Existing Gynecological Surgical Business
- Smartwatches Could Detect Congestive Heart Failure
- Versatile Smart Patch Combines Health Monitoring and Drug Delivery
- Machine Learning Model Improves Mortality Risk Prediction for Cardiac Surgery Patients
- Strategic Collaboration to Develop and Integrate Generative AI into Healthcare
- AI-Enabled Operating Rooms Solution Helps Hospitals Maximize Utilization and Unlock Capacity