Expo
view channel
view channel
view channel
view channel
view channel
Medical Imaging
AICritical Care
Patient CareHealth ITPoint of CareBusiness
Events

- New Approach Monitors Bone Fracture Healing Without X-Ray Radiation
- Modified Smart Microbes Could Treat Inflammatory Bowel Disease
- Wireless, Catheter-Free Device Accurately Monitors Bladder Dysfunction
- Cutting‑Edge Non‑Invasive System Revolutionizes Point-of-Care Hospital Diagnostics
- Dual-Laser Strategy Revolutionizes Breast Cancer Photothermal Therapy
- Surgical Ablation During CABG Improves Survival in Patients with Preexisting Atrial Fibrillation
- New Battery Technology Delivers Additional Power to Implantable Medical Devices
- New Model Reveals Optimal Positioning of Orthopedic Screws in Fractures
- Non-Invasive Tool for Removing Lung Cancer Tumors Reduces Surgical Trauma
- Novel Mechanical Heart Valve Improves Blood Flow and Lowers Risk of Blood Clots
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Next Gen ICU Bed to Help Address Complex Critical Care Needs
- Groundbreaking AI-Powered UV-C Disinfection Technology Redefines Infection Control Landscape
- Expanded Collaboration to Transform OR Technology Through AI and Automation
- Becton Dickinson to Spin Out Biosciences and Diagnostic Solutions Business
- Boston Scientific Acquires Medical Device Company SoniVie
- 2026 World Hospital Congress to be Held in Seoul
- Teleflex to Acquire BIOTRONIK’s Vascular Intervention Business
- Smartwatches Could Detect Congestive Heart Failure
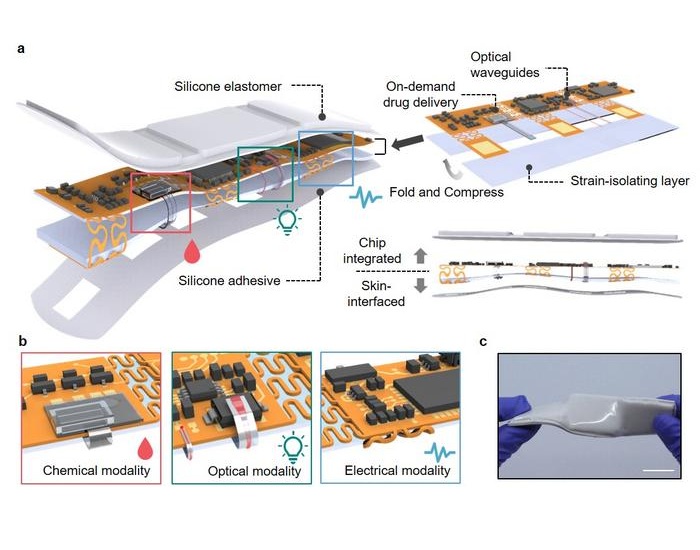
- Versatile Smart Patch Combines Health Monitoring and Drug Delivery
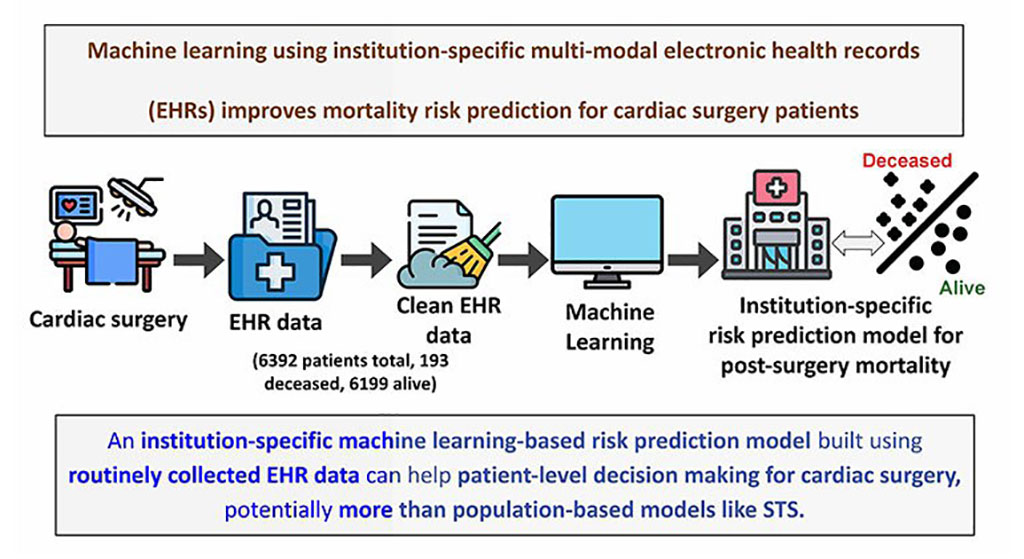
- Machine Learning Model Improves Mortality Risk Prediction for Cardiac Surgery Patients
- Strategic Collaboration to Develop and Integrate Generative AI into Healthcare
- AI-Enabled Operating Rooms Solution Helps Hospitals Maximize Utilization and Unlock Capacity

 Expo
Expo
- New Approach Monitors Bone Fracture Healing Without X-Ray Radiation
- Modified Smart Microbes Could Treat Inflammatory Bowel Disease
- Wireless, Catheter-Free Device Accurately Monitors Bladder Dysfunction
- Cutting‑Edge Non‑Invasive System Revolutionizes Point-of-Care Hospital Diagnostics
- Dual-Laser Strategy Revolutionizes Breast Cancer Photothermal Therapy
- Surgical Ablation During CABG Improves Survival in Patients with Preexisting Atrial Fibrillation
- New Battery Technology Delivers Additional Power to Implantable Medical Devices
- New Model Reveals Optimal Positioning of Orthopedic Screws in Fractures
- Non-Invasive Tool for Removing Lung Cancer Tumors Reduces Surgical Trauma
- Novel Mechanical Heart Valve Improves Blood Flow and Lowers Risk of Blood Clots
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Next Gen ICU Bed to Help Address Complex Critical Care Needs
- Groundbreaking AI-Powered UV-C Disinfection Technology Redefines Infection Control Landscape
- Expanded Collaboration to Transform OR Technology Through AI and Automation
- Becton Dickinson to Spin Out Biosciences and Diagnostic Solutions Business
- Boston Scientific Acquires Medical Device Company SoniVie
- 2026 World Hospital Congress to be Held in Seoul
- Teleflex to Acquire BIOTRONIK’s Vascular Intervention Business
- Smartwatches Could Detect Congestive Heart Failure
- Versatile Smart Patch Combines Health Monitoring and Drug Delivery
- Machine Learning Model Improves Mortality Risk Prediction for Cardiac Surgery Patients
- Strategic Collaboration to Develop and Integrate Generative AI into Healthcare
- AI-Enabled Operating Rooms Solution Helps Hospitals Maximize Utilization and Unlock Capacity