Expo
view channel
view channel
view channel
view channel
Medical Imaging
AICritical Care
Patient CareHealth ITPoint of CareBusiness
Events
Webinars

- New Understanding of Barrett’s Esophagus Formation to Enable Earlier Intervention and Diagnosis
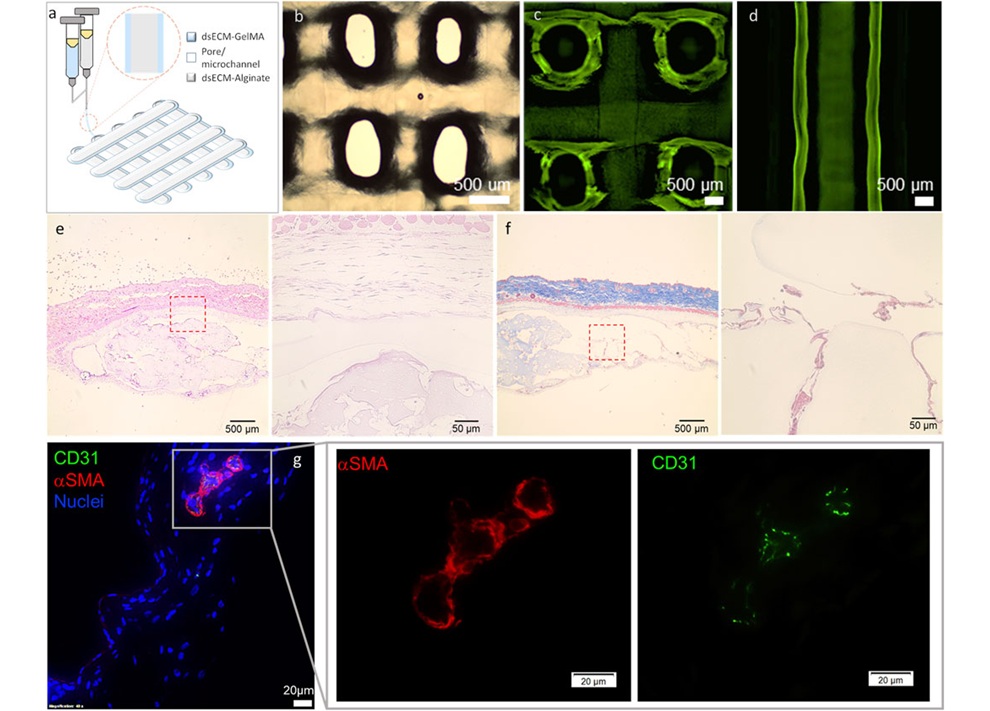
- 3D Printed Functional Human Islets Could Transform Type 1 Diabetes Treatment
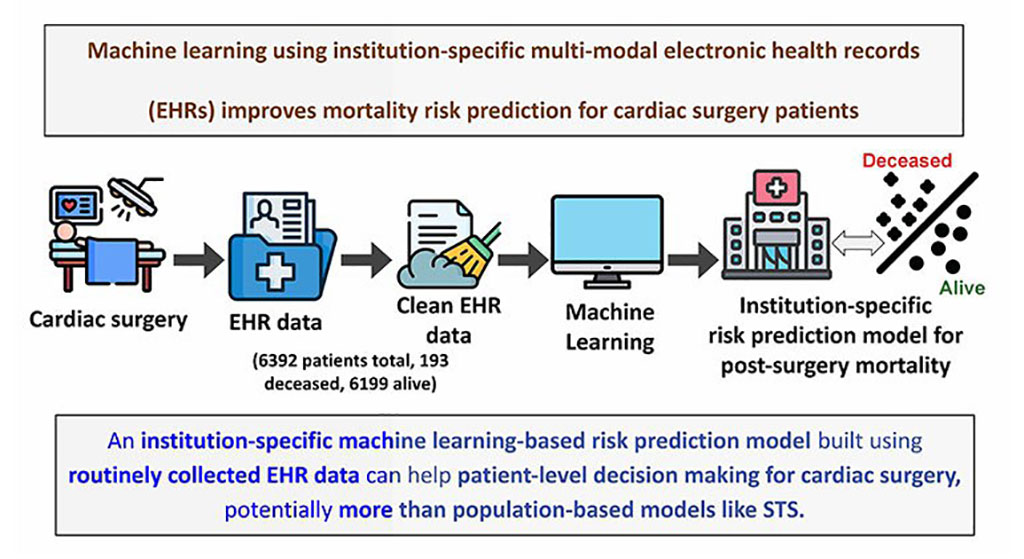
- AI Model Predicts ICU mortality in Heart Failure Patients
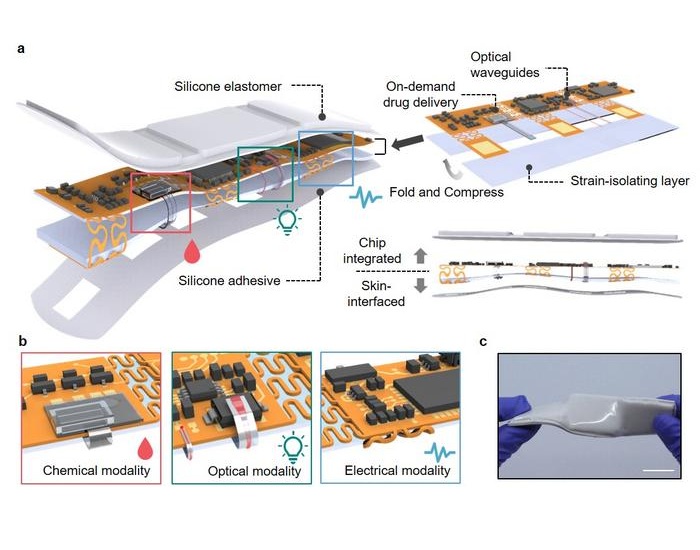
- Ultra-Thin Implant Helps Patients with Spinal Cord Injury Recover Lost Functions
- Smart Capsule Offers Real-Time Profiling Across GI Tract
- First-Ever Technology Makes Blood Translucent During Surgery
- Tibia Nailing System with Novel Side-Specific Nails to Revolutionize Fracture Surgery
- New Imaging Probe to Transform Brain Cancer Surgery
- New Technology More Than Doubles Success Rate for Blood Clot Removal
- Surgical Ablation During CABG Improves Survival in Patients with Preexisting Atrial Fibrillation
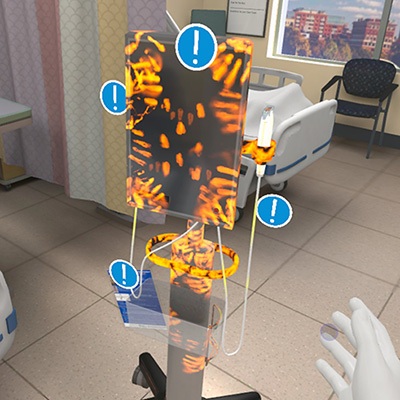
- VR Training Tool Combats Contamination of Portable Medical Equipment
- Portable Biosensor Platform to Reduce Hospital-Acquired Infections
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
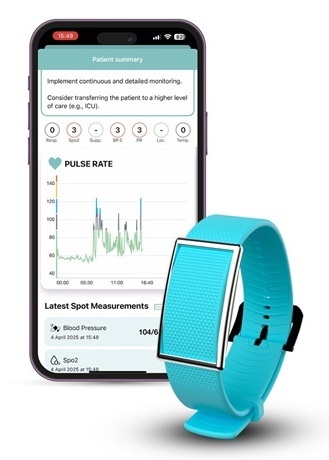
- Medtronic Partners with Corsano to Expand Acute Care & Monitoring Portfolio in Europe
- Expanded Collaboration to Transform OR Technology Through AI and Automation
- Becton Dickinson to Spin Out Biosciences and Diagnostic Solutions Business
- Boston Scientific Acquires Medical Device Company SoniVie
- 2026 World Hospital Congress to be Held in Seoul

 Expo
Expo
- New Understanding of Barrett’s Esophagus Formation to Enable Earlier Intervention and Diagnosis
- 3D Printed Functional Human Islets Could Transform Type 1 Diabetes Treatment
- AI Model Predicts ICU mortality in Heart Failure Patients
- Ultra-Thin Implant Helps Patients with Spinal Cord Injury Recover Lost Functions
- Smart Capsule Offers Real-Time Profiling Across GI Tract
- First-Ever Technology Makes Blood Translucent During Surgery
- Tibia Nailing System with Novel Side-Specific Nails to Revolutionize Fracture Surgery
- New Imaging Probe to Transform Brain Cancer Surgery
- New Technology More Than Doubles Success Rate for Blood Clot Removal
- Surgical Ablation During CABG Improves Survival in Patients with Preexisting Atrial Fibrillation
- VR Training Tool Combats Contamination of Portable Medical Equipment
- Portable Biosensor Platform to Reduce Hospital-Acquired Infections
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Medtronic Partners with Corsano to Expand Acute Care & Monitoring Portfolio in Europe
- Expanded Collaboration to Transform OR Technology Through AI and Automation
- Becton Dickinson to Spin Out Biosciences and Diagnostic Solutions Business
- Boston Scientific Acquires Medical Device Company SoniVie
- 2026 World Hospital Congress to be Held in Seoul