Expo
view channel
view channel
view channel
view channel
view channel
view channel
Medical Imaging
AI
Surgical TechniquesPatient CareHealth ITPoint of CareBusiness
Events

- Pioneering Application Detects Pulse Pressure Using Smartphone
- Mitral Valve Repair Via Catheter Offers Better Outcomes Than Pharmacological Treatment in Heart Failure Patients
- Wearable Lung Patch Accurately Detects Asthma and COPD
- Novel Device-Based Therapies Could Overcome Limitations of Pharmacologic Therapies for Heart Failure Patients
- Wearable Sleep Trackers Could Predict Blood Biomarkers of Alzheimer’s Disease in At-Risk Individuals
- Breakthrough Heart Valve Combines Best of Mechanical and Tissue Replacement Technology
- Ultraportable Battery Powered Medical Device Revolutionizes Concept of Portable Surgical Care
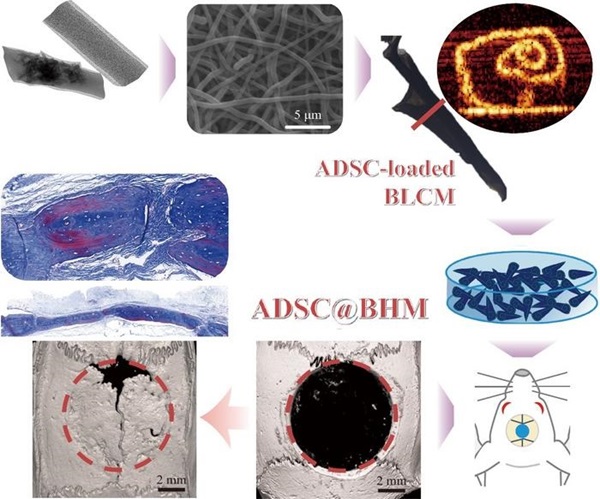
- Groundbreaking Tubular Scaffolds Enhance Bone Regeneration of Critical-Sized Skull Defects
- AI-Powered Precision Surgery Platform to Make Procedures Smarter and Safer
- Novel Magnet Compression Anastomosis Technology Ensures Fewer Complications, Bleeds or Leaks
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Next Gen ICU Bed to Help Address Complex Critical Care Needs
- Groundbreaking AI-Powered UV-C Disinfection Technology Redefines Infection Control Landscape
- Clean Hospitals Can Reduce Antibiotic Resistance, Save Lives
- ZOLL to Acquire Vyaire Medical’s Ventilator Business
- Getinge Acquires Organ Transport Products and Services Company Paragonix Technologies
- Stryker Acquires care.ai to Boost AI-Driven Healthcare
- Johnson & Johnson Acquires Cardiovascular Specialist V-Wave
- Abbott and Medtronic Global Partnership to Integrate Advanced Glucose Sensing Technology with Automated Insulin Delivery Systems
- Strategic Collaboration to Develop and Integrate Generative AI into Healthcare
- AI-Enabled Operating Rooms Solution Helps Hospitals Maximize Utilization and Unlock Capacity
- AI Predicts Pancreatic Cancer Three Years before Diagnosis from Patients’ Medical Records
- First Fully Autonomous Generative AI Personalized Medical Authorizations System Reduces Care Delay
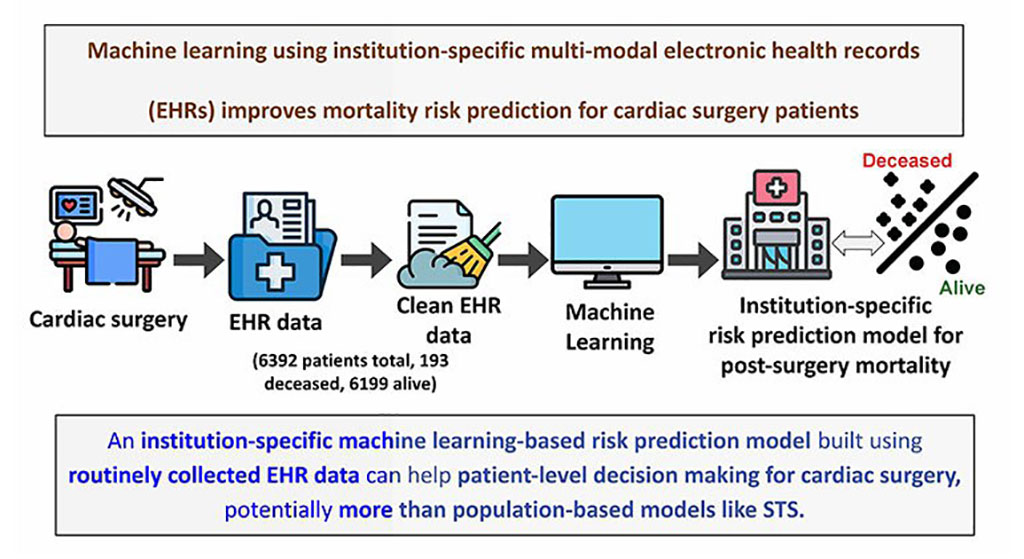
- Electronic Health Records May Be Key to Improving Patient Care, Study Finds
- 5-Minute Multiplex PCR Testing System to Redefine Point-Of-Care Diagnostics
- POCT for Infectious Diseases Delivers Laboratory Equivalent Pathology Results
- Cartridge-Based Hemostasis Analyzer System Enables Faster Coagulation Testing
- Critical Bleeding Management System to Help Hospitals Further Standardize Viscoelastic Testing
- Point of Care HIV Test Enables Early Infection Diagnosis for Infants

Expo
 view channel
view channel
view channel
view channel
view channel
view channel
Medical Imaging
AI
Surgical TechniquesPatient CareHealth ITPoint of CareBusiness
Events
Advertise with Us
view channel
view channel
view channel
view channel
view channel
view channel
Medical Imaging
AI
Surgical TechniquesPatient CareHealth ITPoint of CareBusiness
Events
Advertise with Us


- Pioneering Application Detects Pulse Pressure Using Smartphone
- Mitral Valve Repair Via Catheter Offers Better Outcomes Than Pharmacological Treatment in Heart Failure Patients
- Wearable Lung Patch Accurately Detects Asthma and COPD
- Novel Device-Based Therapies Could Overcome Limitations of Pharmacologic Therapies for Heart Failure Patients
- Wearable Sleep Trackers Could Predict Blood Biomarkers of Alzheimer’s Disease in At-Risk Individuals
- Breakthrough Heart Valve Combines Best of Mechanical and Tissue Replacement Technology
- Ultraportable Battery Powered Medical Device Revolutionizes Concept of Portable Surgical Care
- Groundbreaking Tubular Scaffolds Enhance Bone Regeneration of Critical-Sized Skull Defects
- AI-Powered Precision Surgery Platform to Make Procedures Smarter and Safer
- Novel Magnet Compression Anastomosis Technology Ensures Fewer Complications, Bleeds or Leaks
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Next Gen ICU Bed to Help Address Complex Critical Care Needs
- Groundbreaking AI-Powered UV-C Disinfection Technology Redefines Infection Control Landscape
- Clean Hospitals Can Reduce Antibiotic Resistance, Save Lives
- ZOLL to Acquire Vyaire Medical’s Ventilator Business
- Getinge Acquires Organ Transport Products and Services Company Paragonix Technologies
- Stryker Acquires care.ai to Boost AI-Driven Healthcare
- Johnson & Johnson Acquires Cardiovascular Specialist V-Wave
- Abbott and Medtronic Global Partnership to Integrate Advanced Glucose Sensing Technology with Automated Insulin Delivery Systems
- Strategic Collaboration to Develop and Integrate Generative AI into Healthcare
- AI-Enabled Operating Rooms Solution Helps Hospitals Maximize Utilization and Unlock Capacity
- AI Predicts Pancreatic Cancer Three Years before Diagnosis from Patients’ Medical Records
- First Fully Autonomous Generative AI Personalized Medical Authorizations System Reduces Care Delay
- Electronic Health Records May Be Key to Improving Patient Care, Study Finds
- 5-Minute Multiplex PCR Testing System to Redefine Point-Of-Care Diagnostics
- POCT for Infectious Diseases Delivers Laboratory Equivalent Pathology Results
- Cartridge-Based Hemostasis Analyzer System Enables Faster Coagulation Testing
- Critical Bleeding Management System to Help Hospitals Further Standardize Viscoelastic Testing
- Point of Care HIV Test Enables Early Infection Diagnosis for Infants