Expo
view channel
view channel
view channel
view channel
Medical Imaging
AI
Surgical TechniquesPatient CareHealth ITPoint of CareBusiness
Events
Webinars

- Electrochemical Catheter Hub Prevents Bloodstream Infections
- Noninvasive Double Microbubble Delivery Approach Marks Breakthrough in Brain Cancer Treatment
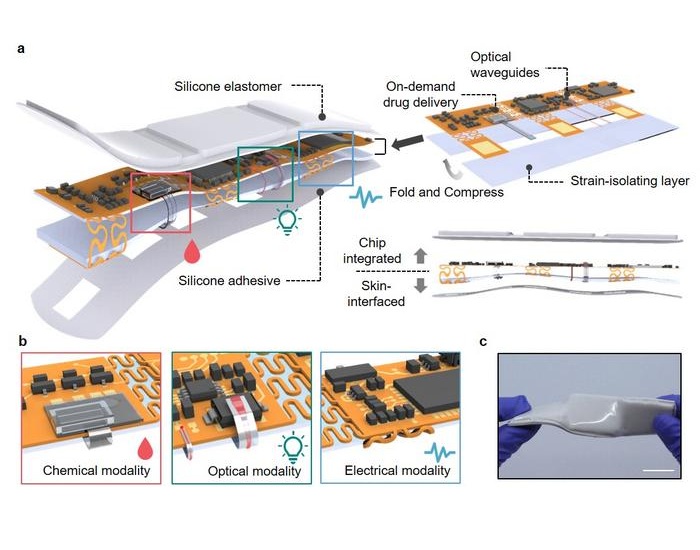
- Self-Healing Skin-Like Material to Find Applications in Health Monitoring, Surgery and Implants
- AI-Powered Wearable Sensor Predicts Labor Onset in Pregnant Women
- Highly-Sensitive Electronic Skin Allows Robots to Feel Heat, Pain and Pressure
- Tibia Nailing System with Novel Side-Specific Nails to Revolutionize Fracture Surgery
- New Imaging Probe to Transform Brain Cancer Surgery
- New Technology More Than Doubles Success Rate for Blood Clot Removal
- Surgical Ablation During CABG Improves Survival in Patients with Preexisting Atrial Fibrillation
- New Battery Technology Delivers Additional Power to Implantable Medical Devices
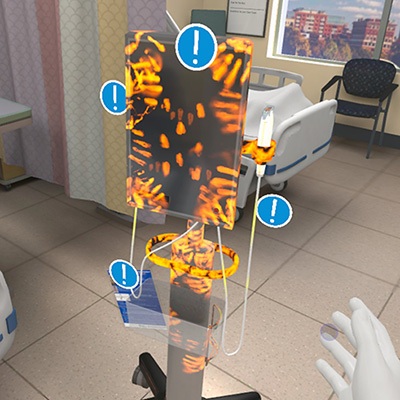
- VR Training Tool Combats Contamination of Portable Medical Equipment
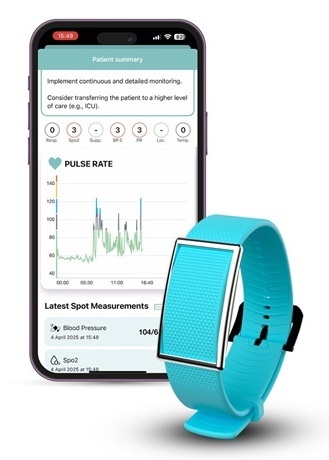
- Portable Biosensor Platform to Reduce Hospital-Acquired Infections
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Medtronic Partners with Corsano to Expand Acute Care & Monitoring Portfolio in Europe
- Expanded Collaboration to Transform OR Technology Through AI and Automation
- Becton Dickinson to Spin Out Biosciences and Diagnostic Solutions Business
- Boston Scientific Acquires Medical Device Company SoniVie
- 2026 World Hospital Congress to be Held in Seoul

 Expo
Expo
- Electrochemical Catheter Hub Prevents Bloodstream Infections
- Noninvasive Double Microbubble Delivery Approach Marks Breakthrough in Brain Cancer Treatment
- Self-Healing Skin-Like Material to Find Applications in Health Monitoring, Surgery and Implants
- AI-Powered Wearable Sensor Predicts Labor Onset in Pregnant Women
- Highly-Sensitive Electronic Skin Allows Robots to Feel Heat, Pain and Pressure
- Tibia Nailing System with Novel Side-Specific Nails to Revolutionize Fracture Surgery
- New Imaging Probe to Transform Brain Cancer Surgery
- New Technology More Than Doubles Success Rate for Blood Clot Removal
- Surgical Ablation During CABG Improves Survival in Patients with Preexisting Atrial Fibrillation
- New Battery Technology Delivers Additional Power to Implantable Medical Devices
- VR Training Tool Combats Contamination of Portable Medical Equipment
- Portable Biosensor Platform to Reduce Hospital-Acquired Infections
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Medtronic Partners with Corsano to Expand Acute Care & Monitoring Portfolio in Europe
- Expanded Collaboration to Transform OR Technology Through AI and Automation
- Becton Dickinson to Spin Out Biosciences and Diagnostic Solutions Business
- Boston Scientific Acquires Medical Device Company SoniVie
- 2026 World Hospital Congress to be Held in Seoul