Expo
view channel
view channel
view channel
view channel
Medical Imaging
AICritical CareSurgical TechniquesPatient CareHealth ITPoint of CareBusiness
Events
Webinars

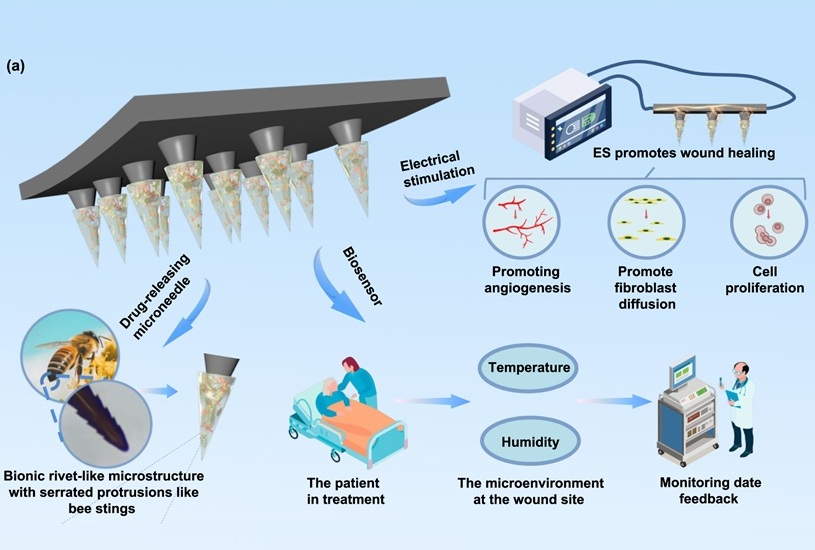
- Bee-Stinger-Inspired Microneedle Delivers Drugs, Stimulates Healing and Monitors Wounds
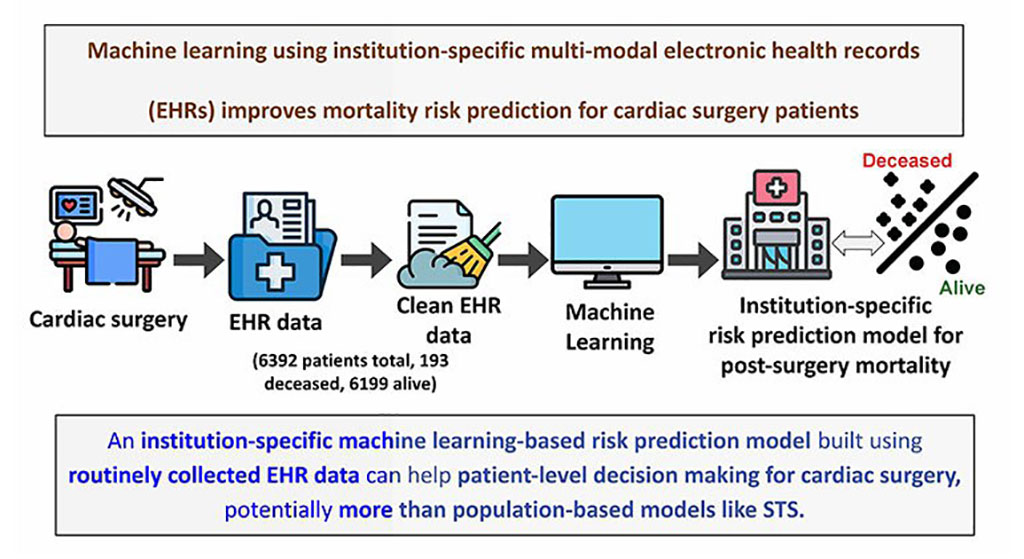
- Blood Markers and ECG Patterns Could Provide Early Warning for Hidden Heart Risks in ICUs
- Multidimensional Diagnostic Approach Identifies Previously Missed At-Risk COPD Patients
- AI Tool Predicts Markers of Alzheimer’s Disease
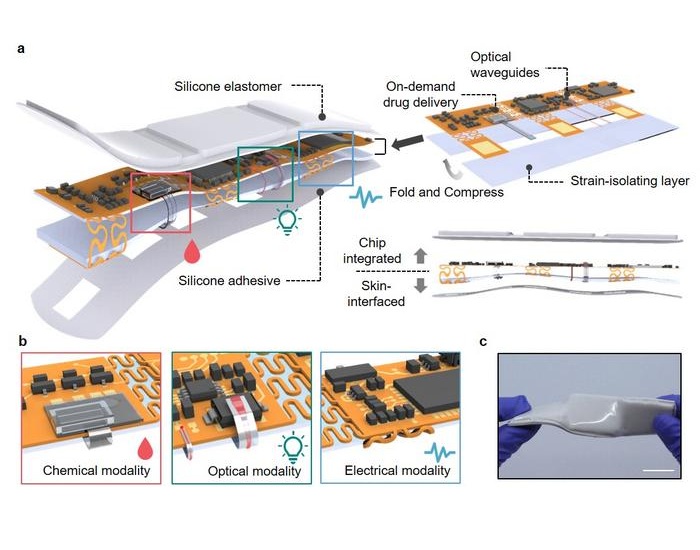
- New Flexible Material Paves Way for Self-Powered Wearable Sensors
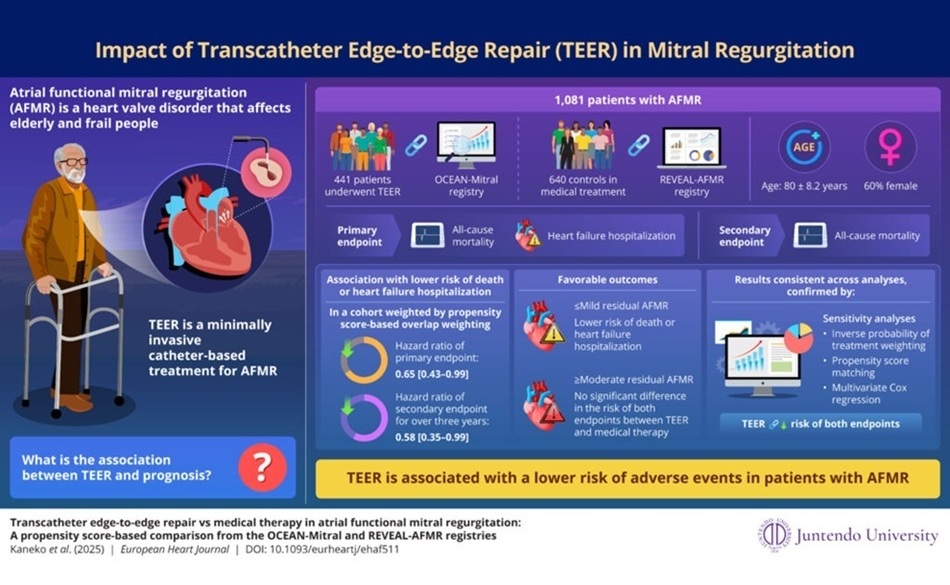
- Minimally Invasive Valve Repair Improves Survival in Elderly AFMR Patients
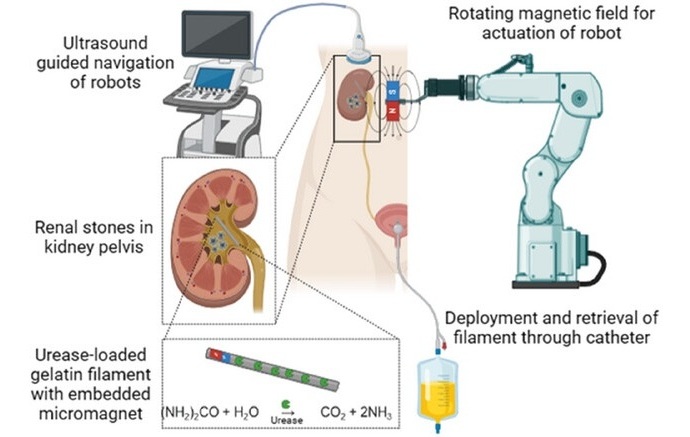
- Tiny Soft Robots Dissolve Painful Kidney Stones with Targeted Drug Delivery
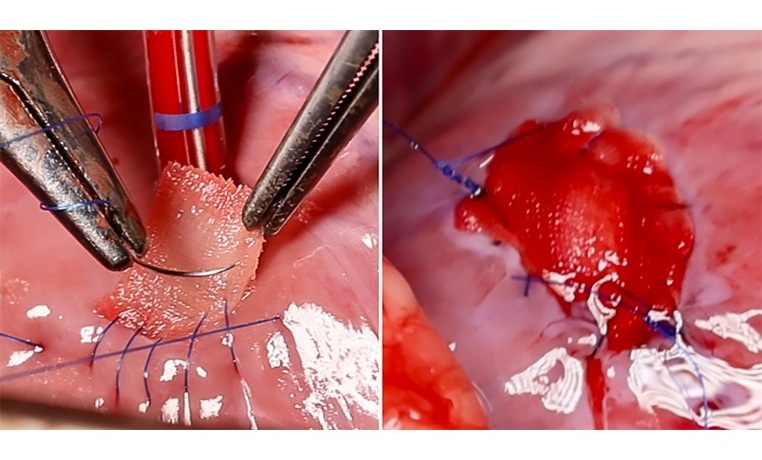
- Implantable 3D Patch Closes and Repairs Heart Defects
- New Endoscopy Technology Enables Early Detection of Esophageal Cancer
- New Implant Enables Women to Access Hip Resurfacing Surgery
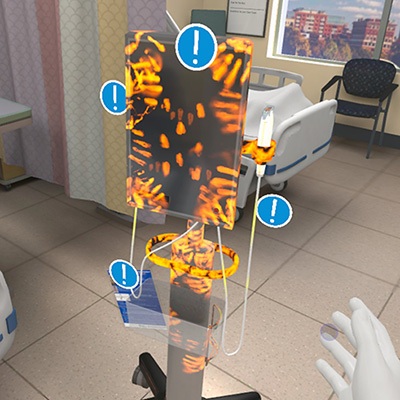
- VR Training Tool Combats Contamination of Portable Medical Equipment
- Portable Biosensor Platform to Reduce Hospital-Acquired Infections
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Medtronic Partners with Corsano to Expand Acute Care & Monitoring Portfolio in Europe
- Expanded Collaboration to Transform OR Technology Through AI and Automation
- Becton Dickinson to Spin Out Biosciences and Diagnostic Solutions Business
- Boston Scientific Acquires Medical Device Company SoniVie
- 2026 World Hospital Congress to be Held in Seoul

 Expo
Expo
- Bee-Stinger-Inspired Microneedle Delivers Drugs, Stimulates Healing and Monitors Wounds
- Blood Markers and ECG Patterns Could Provide Early Warning for Hidden Heart Risks in ICUs
- Multidimensional Diagnostic Approach Identifies Previously Missed At-Risk COPD Patients
- AI Tool Predicts Markers of Alzheimer’s Disease
- New Flexible Material Paves Way for Self-Powered Wearable Sensors
- Minimally Invasive Valve Repair Improves Survival in Elderly AFMR Patients
- Tiny Soft Robots Dissolve Painful Kidney Stones with Targeted Drug Delivery
- Implantable 3D Patch Closes and Repairs Heart Defects
- New Endoscopy Technology Enables Early Detection of Esophageal Cancer
- New Implant Enables Women to Access Hip Resurfacing Surgery
- VR Training Tool Combats Contamination of Portable Medical Equipment
- Portable Biosensor Platform to Reduce Hospital-Acquired Infections
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Medtronic Partners with Corsano to Expand Acute Care & Monitoring Portfolio in Europe
- Expanded Collaboration to Transform OR Technology Through AI and Automation
- Becton Dickinson to Spin Out Biosciences and Diagnostic Solutions Business
- Boston Scientific Acquires Medical Device Company SoniVie
- 2026 World Hospital Congress to be Held in Seoul
















.jpg)