Expo
view channel
view channel
view channel
view channel
Medical Imaging
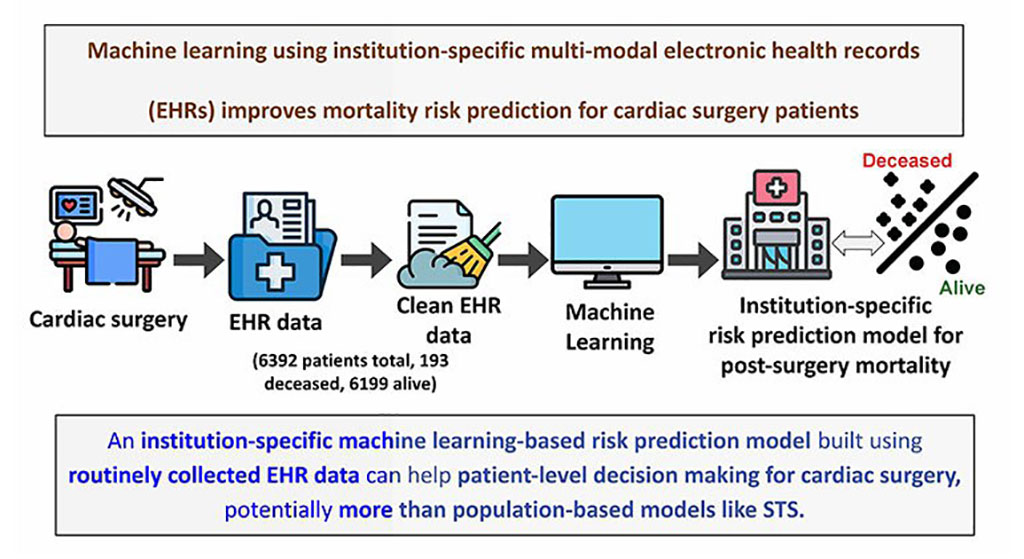
AI
Surgical TechniquesPatient CareHealth ITPoint of CareBusiness
Events
Webinars

- Highly-Sensitive Electronic Skin Allows Robots to Feel Heat, Pain and Pressure
- Implantable Device to Redefine Continuous Glucose Monitoring
- Smart Microgel Could Repair and Replace Damaged Organs
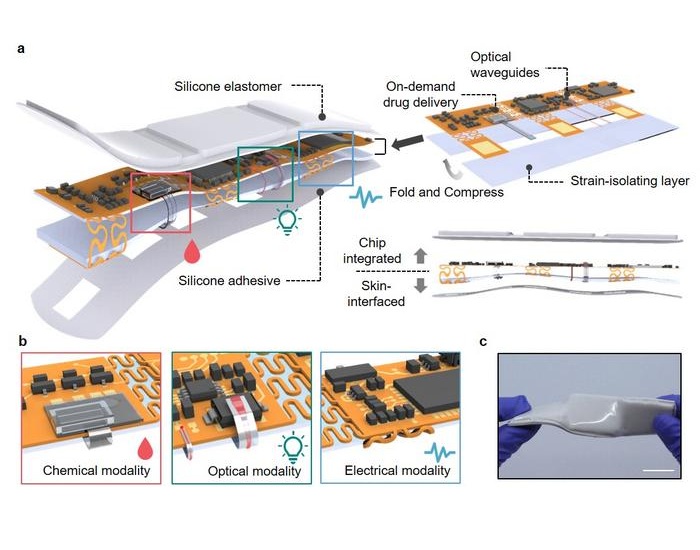
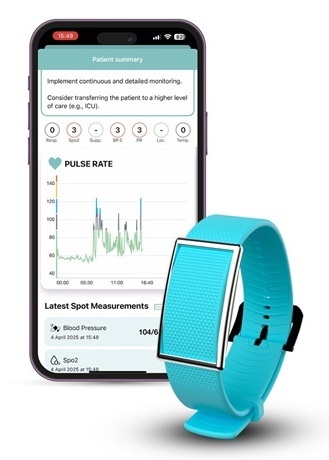
- Smart Breath Tracker Wristband to Revolutionize Respiratory Care
- Stronger Blood Clot Prevention Measures Needed After Leg Artery Procedures in High-Risk Patients
- New Imaging Probe to Transform Brain Cancer Surgery
- New Technology More Than Doubles Success Rate for Blood Clot Removal
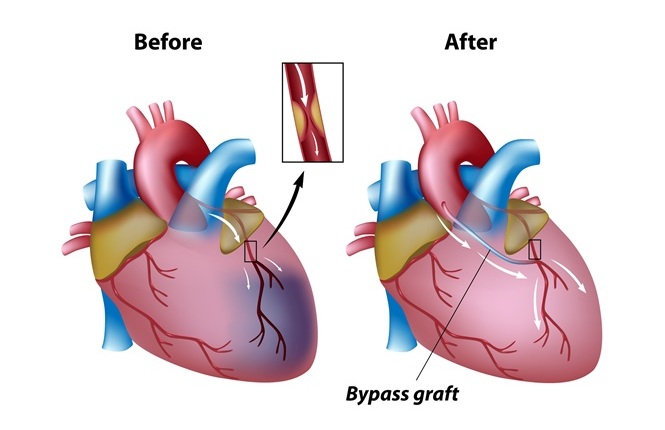
- Surgical Ablation During CABG Improves Survival in Patients with Preexisting Atrial Fibrillation
- New Battery Technology Delivers Additional Power to Implantable Medical Devices
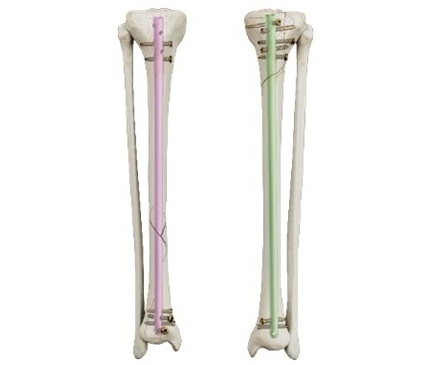
- New Model Reveals Optimal Positioning of Orthopedic Screws in Fractures
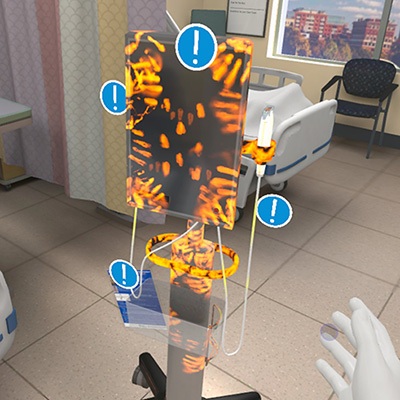
- VR Training Tool Combats Contamination of Portable Medical Equipment
- Portable Biosensor Platform to Reduce Hospital-Acquired Infections
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Medtronic Partners with Corsano to Expand Acute Care & Monitoring Portfolio in Europe
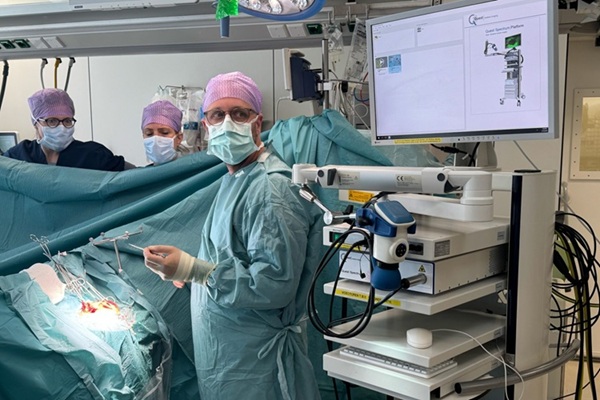
- Expanded Collaboration to Transform OR Technology Through AI and Automation
- Becton Dickinson to Spin Out Biosciences and Diagnostic Solutions Business
- Boston Scientific Acquires Medical Device Company SoniVie
- 2026 World Hospital Congress to be Held in Seoul

 Expo
Expo
- Highly-Sensitive Electronic Skin Allows Robots to Feel Heat, Pain and Pressure
- Implantable Device to Redefine Continuous Glucose Monitoring
- Smart Microgel Could Repair and Replace Damaged Organs
- Smart Breath Tracker Wristband to Revolutionize Respiratory Care
- Stronger Blood Clot Prevention Measures Needed After Leg Artery Procedures in High-Risk Patients
- New Imaging Probe to Transform Brain Cancer Surgery
- New Technology More Than Doubles Success Rate for Blood Clot Removal
- Surgical Ablation During CABG Improves Survival in Patients with Preexisting Atrial Fibrillation
- New Battery Technology Delivers Additional Power to Implantable Medical Devices
- New Model Reveals Optimal Positioning of Orthopedic Screws in Fractures
- VR Training Tool Combats Contamination of Portable Medical Equipment
- Portable Biosensor Platform to Reduce Hospital-Acquired Infections
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Medtronic Partners with Corsano to Expand Acute Care & Monitoring Portfolio in Europe
- Expanded Collaboration to Transform OR Technology Through AI and Automation
- Becton Dickinson to Spin Out Biosciences and Diagnostic Solutions Business
- Boston Scientific Acquires Medical Device Company SoniVie
- 2026 World Hospital Congress to be Held in Seoul