Expo
view channel
view channel
view channel
view channel
view channel
Medical Imaging
AI
Surgical TechniquesPatient CareHealth ITPoint of CareBusiness
Events

- Breakthrough Sensor Technology Tracks Stroke After Effects
- New Study Demonstrates AI-Assisted Detection of Reduced Ejection Fraction
- Novel 3D Adipose Tissue Bioprinting Method to Find Applications in Regenerative Medicine
- Miniaturized Pacemaker for Newborns Found Safe and Effective for Up to Two Years
- World’s First 3D Neural Electrode Uses Soft Actuation Technology to Avoid Nerve Damage
- Screw-Shaped Magnetic Microrobots to Transform Treatment for Patients with Inoperable Blood Clots
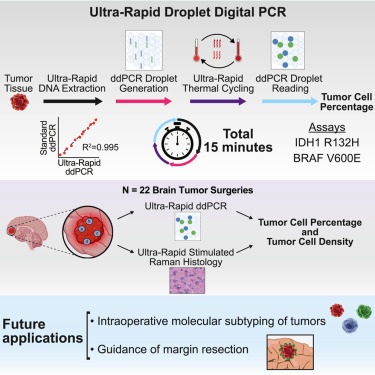
- "Ultra-Rapid" Testing in the OR Could Enable Accurate Removal of Brain Tumors
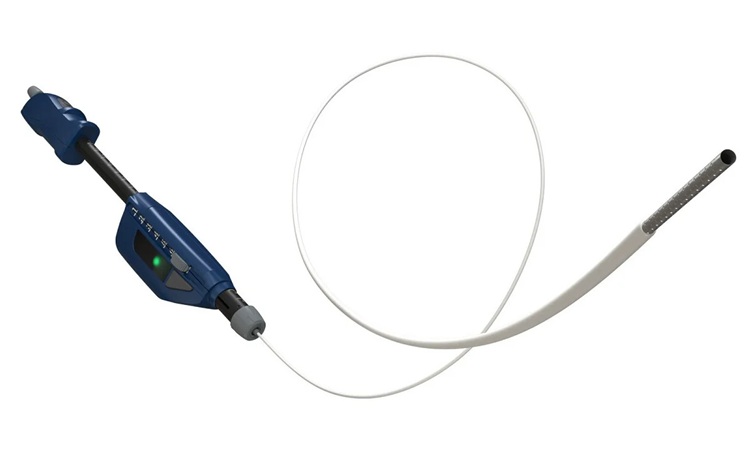
- Automated Endoscopic Device Obtains Improved Biopsy Results in Single Pass
- World's First Machine Learning Model Combats Wrong-Site Surgery
- Novel Method Combining Heart Biopsy and Device Implantation Reduces Complications Risk
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Next Gen ICU Bed to Help Address Complex Critical Care Needs
- Groundbreaking AI-Powered UV-C Disinfection Technology Redefines Infection Control Landscape
- Boston Scientific Acquires Medical Device Company SoniVie
- 2026 World Hospital Congress to be Held in Seoul
- Teleflex to Acquire BIOTRONIK’s Vascular Intervention Business
- Philips and Mass General Brigham Collaborate on Improving Patient Care with Live AI-Powered Insights
- Arab Health 2025 Celebrates Landmark 50th Edition
- Smartwatches Could Detect Congestive Heart Failure
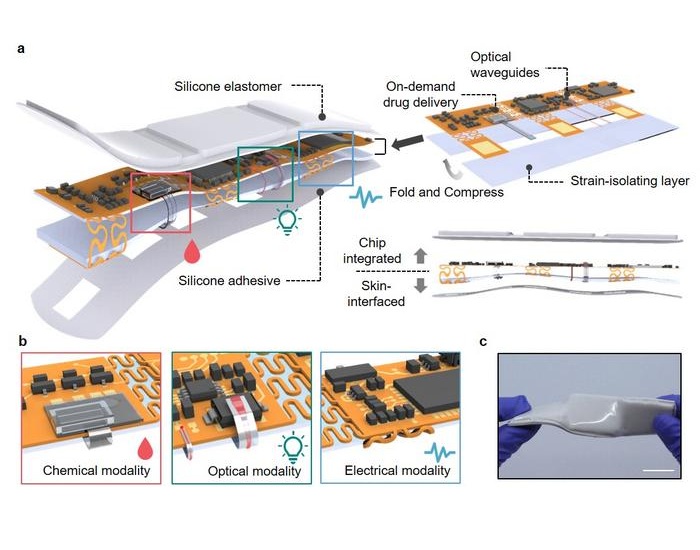
- Versatile Smart Patch Combines Health Monitoring and Drug Delivery
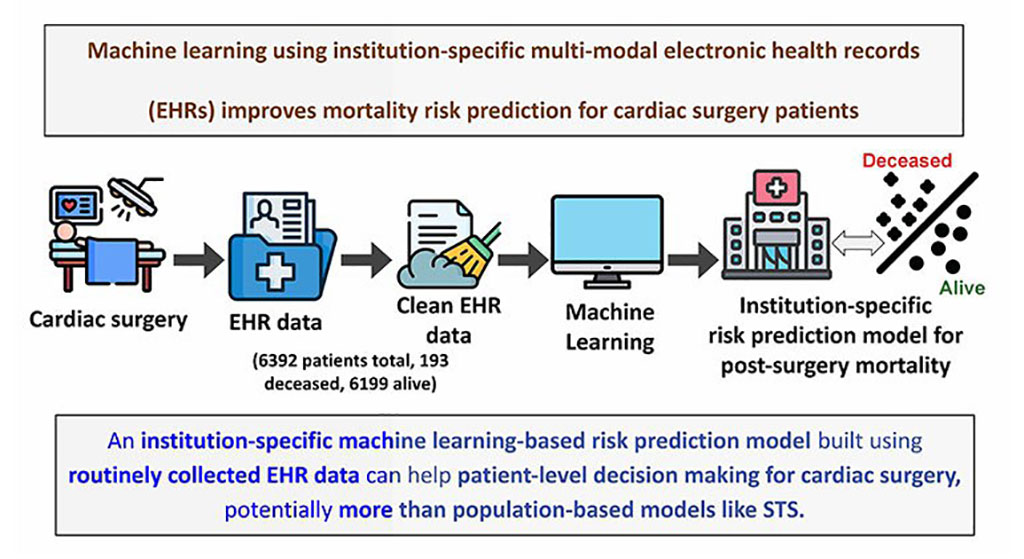
- Machine Learning Model Improves Mortality Risk Prediction for Cardiac Surgery Patients
- Strategic Collaboration to Develop and Integrate Generative AI into Healthcare
- AI-Enabled Operating Rooms Solution Helps Hospitals Maximize Utilization and Unlock Capacity

Expo
 view channel
view channel
view channel
view channel
view channel
Medical Imaging
AI
Surgical TechniquesPatient CareHealth ITPoint of CareBusiness
Events
Advertise with Us
view channel
view channel
view channel
view channel
view channel
Medical Imaging
AI
Surgical TechniquesPatient CareHealth ITPoint of CareBusiness
Events
Advertise with Us


- Breakthrough Sensor Technology Tracks Stroke After Effects
- New Study Demonstrates AI-Assisted Detection of Reduced Ejection Fraction
- Novel 3D Adipose Tissue Bioprinting Method to Find Applications in Regenerative Medicine
- Miniaturized Pacemaker for Newborns Found Safe and Effective for Up to Two Years
- World’s First 3D Neural Electrode Uses Soft Actuation Technology to Avoid Nerve Damage
- Screw-Shaped Magnetic Microrobots to Transform Treatment for Patients with Inoperable Blood Clots
- "Ultra-Rapid" Testing in the OR Could Enable Accurate Removal of Brain Tumors
- Automated Endoscopic Device Obtains Improved Biopsy Results in Single Pass
- World's First Machine Learning Model Combats Wrong-Site Surgery
- Novel Method Combining Heart Biopsy and Device Implantation Reduces Complications Risk
- First-Of-Its-Kind Portable Germicidal Light Technology Disinfects High-Touch Clinical Surfaces in Seconds
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Next Gen ICU Bed to Help Address Complex Critical Care Needs
- Groundbreaking AI-Powered UV-C Disinfection Technology Redefines Infection Control Landscape
- Boston Scientific Acquires Medical Device Company SoniVie
- 2026 World Hospital Congress to be Held in Seoul
- Teleflex to Acquire BIOTRONIK’s Vascular Intervention Business
- Philips and Mass General Brigham Collaborate on Improving Patient Care with Live AI-Powered Insights
- Arab Health 2025 Celebrates Landmark 50th Edition
- Smartwatches Could Detect Congestive Heart Failure
- Versatile Smart Patch Combines Health Monitoring and Drug Delivery
- Machine Learning Model Improves Mortality Risk Prediction for Cardiac Surgery Patients
- Strategic Collaboration to Develop and Integrate Generative AI into Healthcare
- AI-Enabled Operating Rooms Solution Helps Hospitals Maximize Utilization and Unlock Capacity