Expo
view channel
view channel
view channel
view channel
view channel
view channel
Medical Imaging
AI
Surgical TechniquesPatient CareHealth ITPoint of CareBusiness
Events

- Intelligent Brain Pacemaker to Help Reduce Parkinson’s Disease Symptoms
- Revolutionary Hemostatic Gel Technology Treats Penetrating Traumatic Brain Injury
- New System with Insertable Biosensor to Revolutionize Continuous Glucose Monitoring
- Air-Powered Computer to Help Prevent Blood Clots and Strokes
- Machine Learning Detects Cardiovascular Diseases Before Symptoms Appear
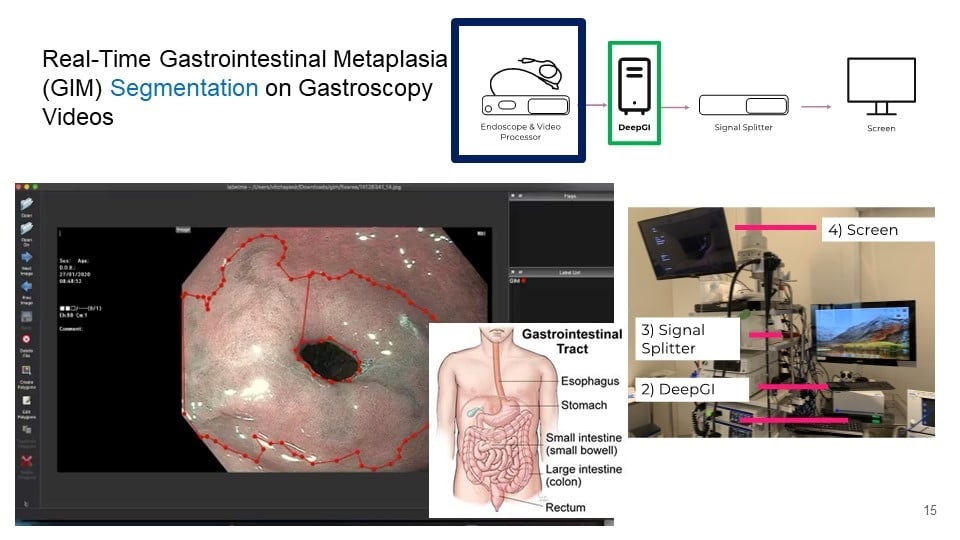
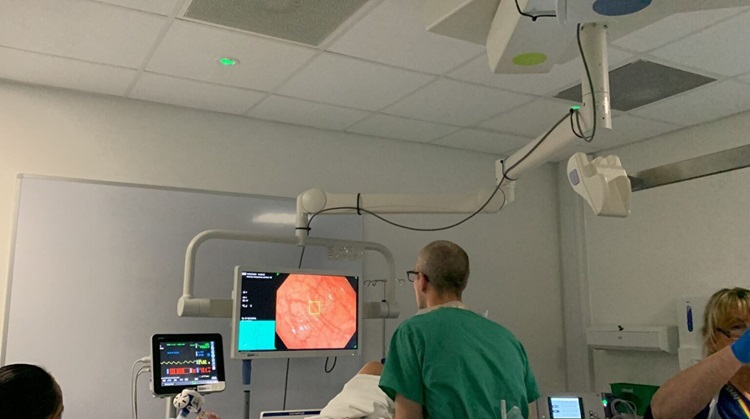
- AI in Colonoscopies Detects Early Signs of Bowel Cancer
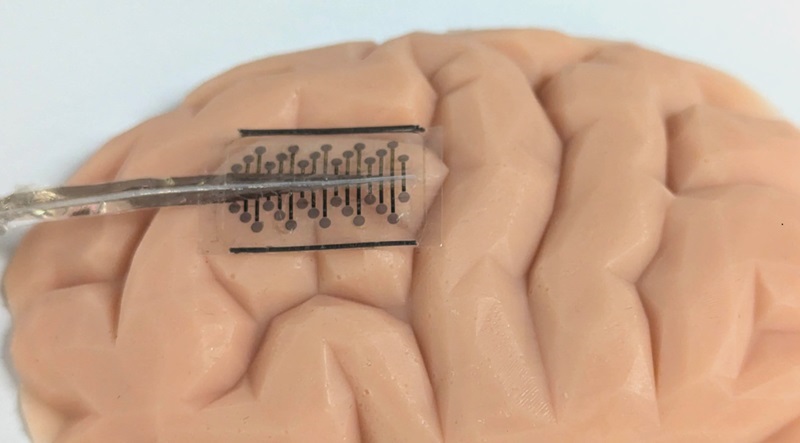
- Tiny Folding Implants to Reduce Surgery for Treatment of Brain Conditions
- Nanosecond Pulsed Field Ablation Technology Effective in Treating Atrial Fibrillation in Cardiac Surgery
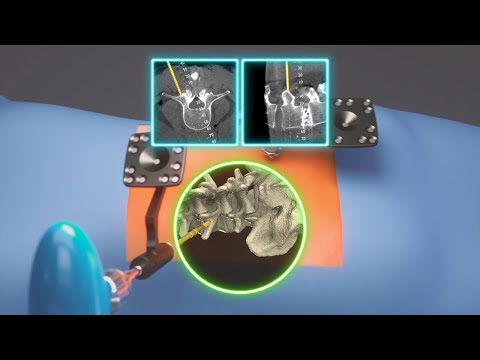
- Active Spine Robotics and Navigation Platform Returns Control to Surgeon's Hands
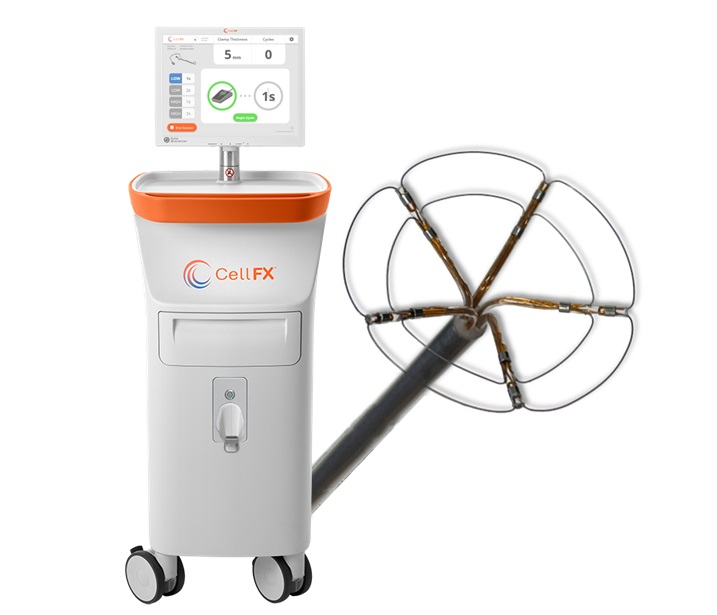
- Groundbreaking Vine Robots with Magnetic Skin to Transform Cancer Treatment
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
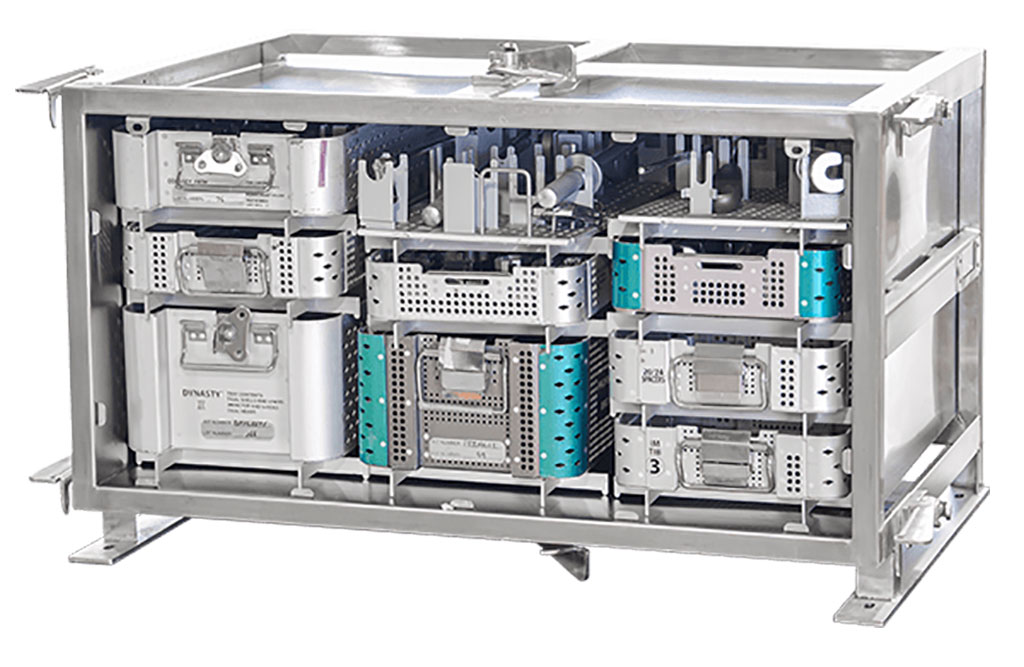
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
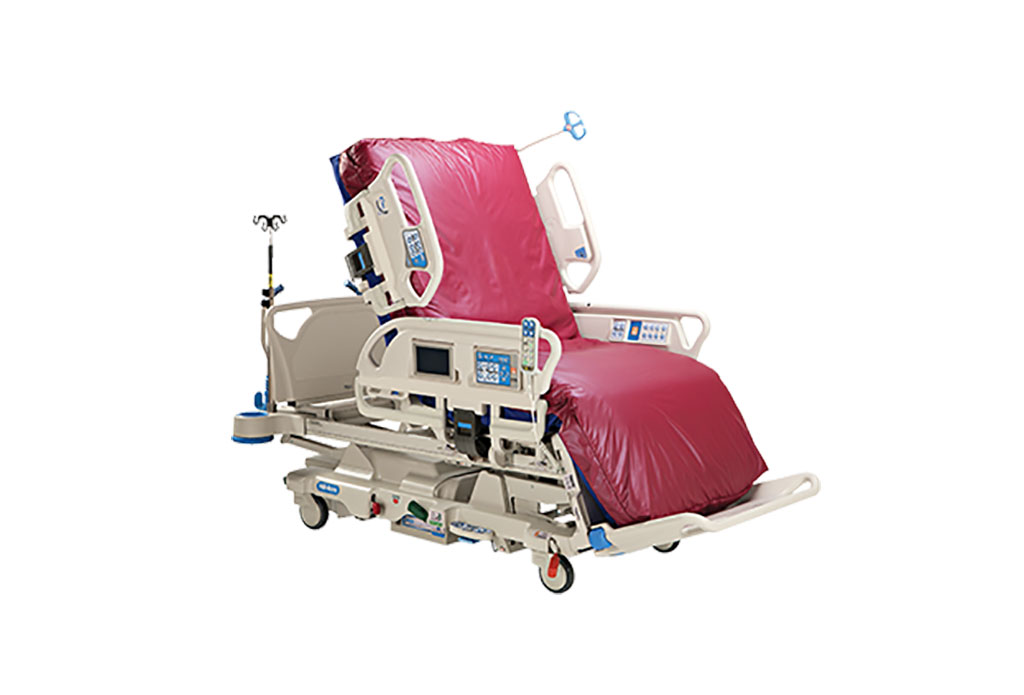
- Next Gen ICU Bed to Help Address Complex Critical Care Needs
- Groundbreaking AI-Powered UV-C Disinfection Technology Redefines Infection Control Landscape
- Clean Hospitals Can Reduce Antibiotic Resistance, Save Lives
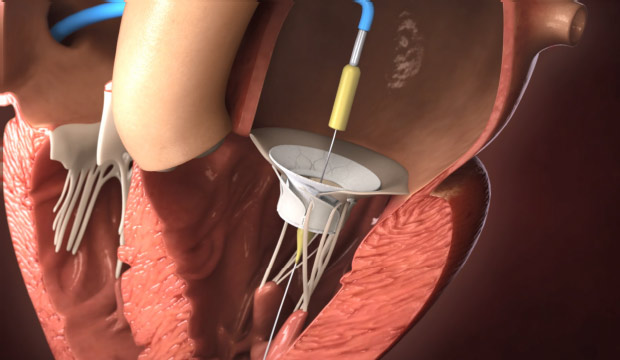
- Edwards Lifesciences Acquires Sheba Medical’s Innovalve Bio Medical
- International Hospital Federation Awards 2024 Finalists Announced
- 2024 World Medical Tourism Conference and Medical Tourism Expo to Showcase Latest Innovations
- BD Acquires Edwards Lifesciences' Critical Care Product Group for USD 4.2 Billion
- MEDICA INNOVATION FORUM for the Healthcare Innovations of the Future
- Strategic Collaboration to Develop and Integrate Generative AI into Healthcare
- AI-Enabled Operating Rooms Solution Helps Hospitals Maximize Utilization and Unlock Capacity
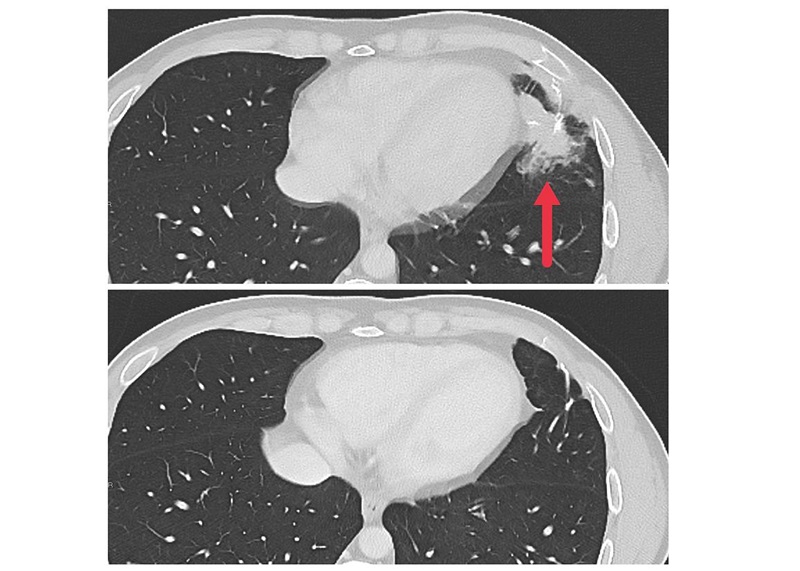
- AI Predicts Pancreatic Cancer Three Years before Diagnosis from Patients’ Medical Records
- First Fully Autonomous Generative AI Personalized Medical Authorizations System Reduces Care Delay
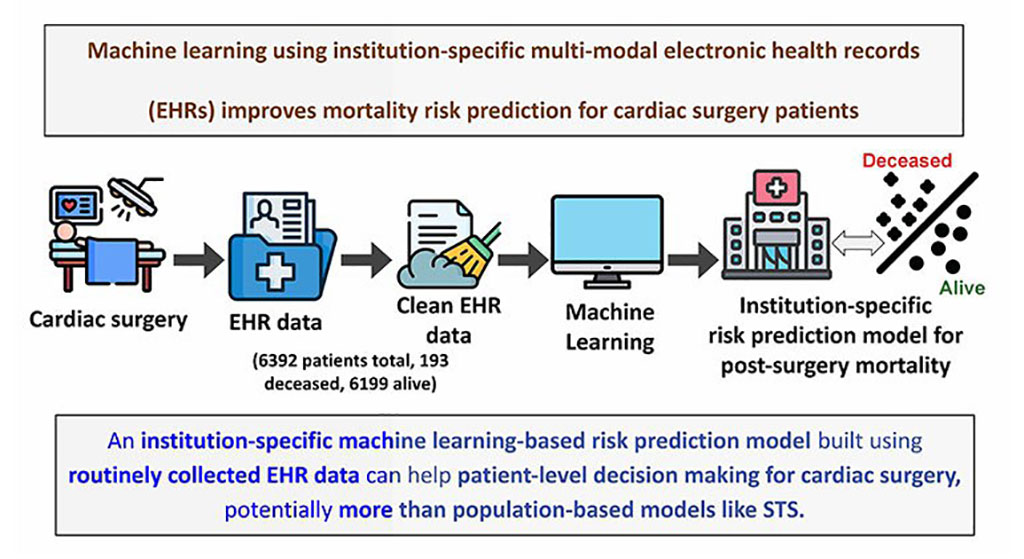
- Electronic Health Records May Be Key to Improving Patient Care, Study Finds
- POCT for Infectious Diseases Delivers Laboratory Equivalent Pathology Results
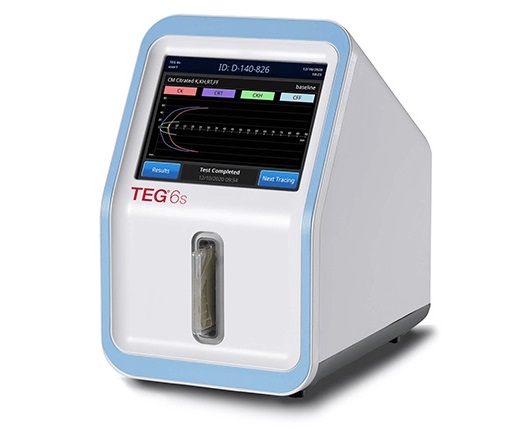
- Cartridge-Based Hemostasis Analyzer System Enables Faster Coagulation Testing
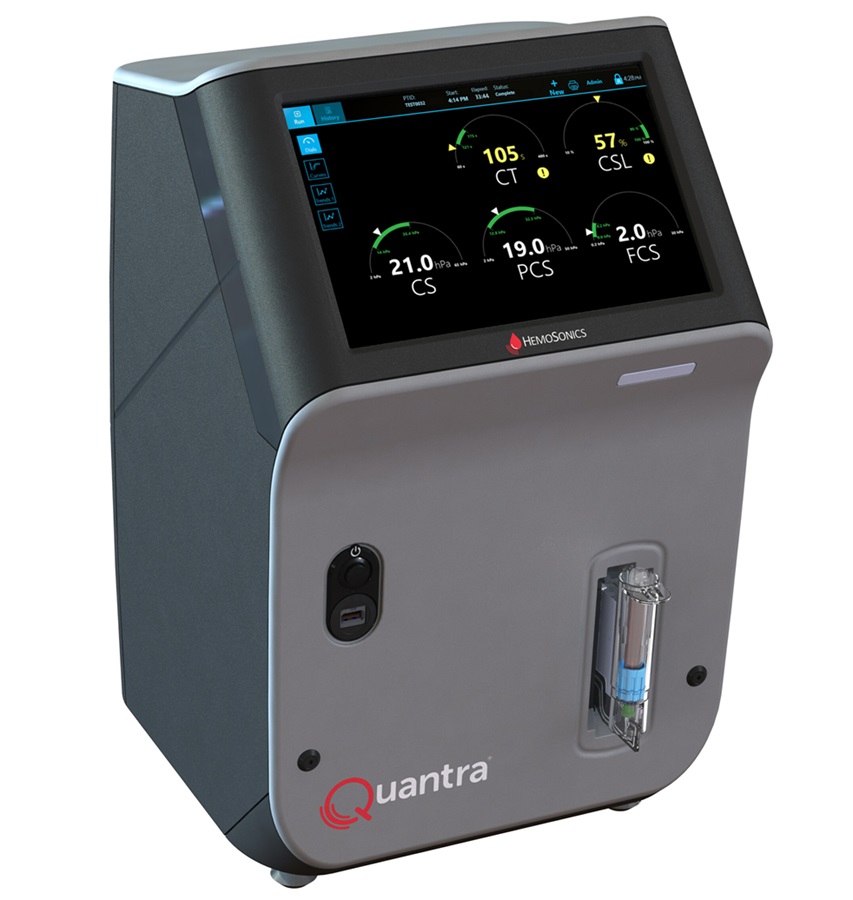
- Critical Bleeding Management System to Help Hospitals Further Standardize Viscoelastic Testing
- Point of Care HIV Test Enables Early Infection Diagnosis for Infants
- Whole Blood Rapid Test Aids Assessment of Concussion at Patient's Bedside

Expo
 view channel
view channel
view channel
view channel
view channel
view channel
Medical Imaging
AI
Surgical TechniquesPatient CareHealth ITPoint of CareBusiness
Events
Advertise with Us
view channel
view channel
view channel
view channel
view channel
view channel
Medical Imaging
AI
Surgical TechniquesPatient CareHealth ITPoint of CareBusiness
Events
Advertise with Us


- Intelligent Brain Pacemaker to Help Reduce Parkinson’s Disease Symptoms
- Revolutionary Hemostatic Gel Technology Treats Penetrating Traumatic Brain Injury
- New System with Insertable Biosensor to Revolutionize Continuous Glucose Monitoring
- Air-Powered Computer to Help Prevent Blood Clots and Strokes
- Machine Learning Detects Cardiovascular Diseases Before Symptoms Appear
- AI in Colonoscopies Detects Early Signs of Bowel Cancer
- Tiny Folding Implants to Reduce Surgery for Treatment of Brain Conditions
- Nanosecond Pulsed Field Ablation Technology Effective in Treating Atrial Fibrillation in Cardiac Surgery
- Active Spine Robotics and Navigation Platform Returns Control to Surgeon's Hands
- Groundbreaking Vine Robots with Magnetic Skin to Transform Cancer Treatment
- Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization
- Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput
- Next Gen ICU Bed to Help Address Complex Critical Care Needs
- Groundbreaking AI-Powered UV-C Disinfection Technology Redefines Infection Control Landscape
- Clean Hospitals Can Reduce Antibiotic Resistance, Save Lives
- Edwards Lifesciences Acquires Sheba Medical’s Innovalve Bio Medical
- International Hospital Federation Awards 2024 Finalists Announced
- 2024 World Medical Tourism Conference and Medical Tourism Expo to Showcase Latest Innovations
- BD Acquires Edwards Lifesciences' Critical Care Product Group for USD 4.2 Billion
- MEDICA INNOVATION FORUM for the Healthcare Innovations of the Future
- Strategic Collaboration to Develop and Integrate Generative AI into Healthcare
- AI-Enabled Operating Rooms Solution Helps Hospitals Maximize Utilization and Unlock Capacity
- AI Predicts Pancreatic Cancer Three Years before Diagnosis from Patients’ Medical Records
- First Fully Autonomous Generative AI Personalized Medical Authorizations System Reduces Care Delay
- Electronic Health Records May Be Key to Improving Patient Care, Study Finds
- POCT for Infectious Diseases Delivers Laboratory Equivalent Pathology Results
- Cartridge-Based Hemostasis Analyzer System Enables Faster Coagulation Testing
- Critical Bleeding Management System to Help Hospitals Further Standardize Viscoelastic Testing
- Point of Care HIV Test Enables Early Infection Diagnosis for Infants
- Whole Blood Rapid Test Aids Assessment of Concussion at Patient's Bedside